How Many Neutrons Does Magnesium Have?
Magnesium has 12 neutrons.
How to Find the Number of Neutrons in Magnesium
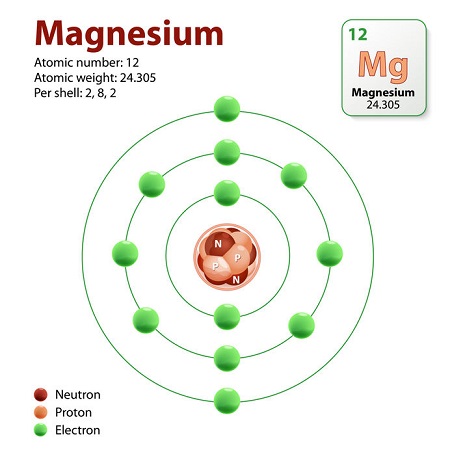
To find the number of neutrons for any element, find the element on the periodic table of elements to get its atomic number and atomic mass. The atomic number is equal to the number of protons. Atomic mass minus the number of protons equals the number of neutrons. For example, the atomic number for magnesium is equal to 12, and the atomic mass is equal to 24, so to find the number of neutrons, subtract 12 from 24 to get 12.
Magnesium in Industry

In industry, magnesium is used in pyrotechnics and incendiary devices. In addition, magnesium was once used in photographic flash powders. Magnesium alloys are frequently used to strengthen weld joints. Because magnesium alloys are lighter than aluminum, they are frequently found in aircraft, automobile, and missile construction.
Magnesium in Pharmacology

Pharmacological uses for magnesium include magnesium hydroxide, used in milk of magnesia. Magnesium sulfate, or Epsom salts, can be used both medicinally and in horticultural. Sprinkled around the base of plants, such as tomatoes, Epsom salts provide magnesium directly to the root system and aid in proper growth and development. Magnesium is essential to life as we know it and is present in all body tissues, where it is responsible for energy transfer.
Resources