How Many Protons Are In Nitrogen?
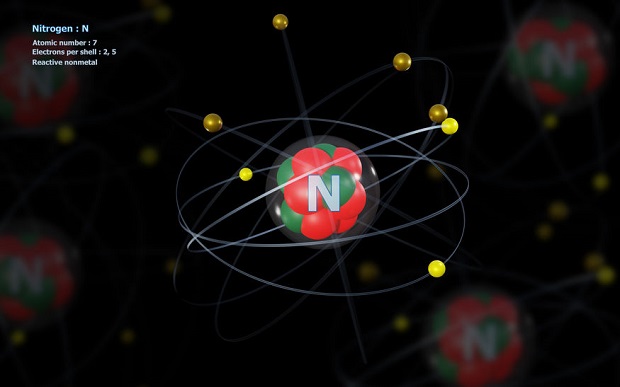
Nitrogen has seven protons, 2 protons on the 1st shell and 5 protons on the second. The periodic table has several categorizations for its elements, and one of these is the atomic number. The atomic number communicates how many protons an element possesses. One atom of nitrogen will always have seven protons. Likewise, if you find an atom with seven protons in its nucleus, it will always be nitrogen. There are no overlaps in the atomic numbering system.
What Is an Atom’s Nucleus?

An atom is a term for a single unit of an element. Atoms compose every physical object in the world, including your chair, your beverage, and the atmosphere you breathe. An atom possesses several important characteristics, one of the most important of which is its nucleus. The nucleus of an atom is the centermost part. It is composed of several particles known as protons and neutrons.
What Are Protons?
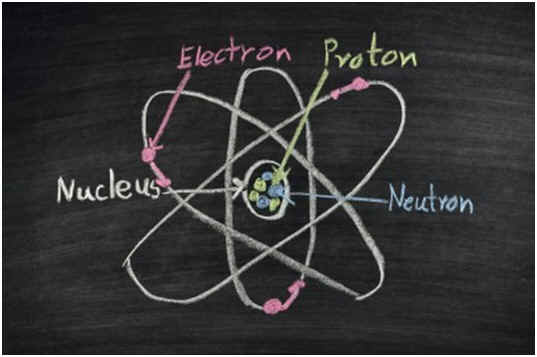
Protons are particles with a positive charge. They bundle together in the nucleus along with neutrons. Their positive charge attracts electrons, which are found in the atom’s outer shell layers. Electrons are negatively charged particles and often interact with the outer shells of other atoms to form chemical compounds. However, the number of electrons that can be held or bonded is ultimately dependent upon the number of protons.
What about Other Forms of Nitrogen?

The atomic number is an element’s one reliable identifier. Although elements can appear in all the states of matter, they will always retain the same number of protons. States of matter involve a change in temperature and/or pressure, not atomic variations. Although various isotopes of nitrogen can be found in nature or made in the laboratory, they each have the same number of protons. Isotopes come about from changing the number of neutrons in the nucleus, not the number of protons. Although nitrogen can form chemical compounds with other elements, such as bonding with potassium to form potassium nitrate, it does so by sharing electrons, not by changing protons.
References
Los Alamos National Laboratory – “Periodic Table of Elements: Nitrogen“
NIST – “Periodic Table of the Elements“
NDT Resource Center – “Subatomic Particles“
Frostburg State University – Chemistry Department – “What Is an Isotope?“
Glossary of Terms
Electron: an elementary particle consisting of a charge of negative electricity equal to about 1.602 × 10−19 coulomb and having a mass when at rest of about 9.109 × 10−31 kilogram or about 1⁄1836 that of a proton.
Merriam-Webster Dictionary
Isotope: Atoms of the same element can have different numbers of neutrons; the different possible versions of each element are called isotopes.
Colorado State University
Nucleus: the positively-charged center of an atom that usually contains the protons and neutrons.
Biology-online
Proton: an elementary particle that is identical with the nucleus of the hydrogen atom, that along with the neutron is a constituent of all other atomic nuclei, that carries a positive charge numerically equal to the charge of an electron, and that has a mass of 1.673 × 10−27 kilogram.
Merriam-Webster Dictionary