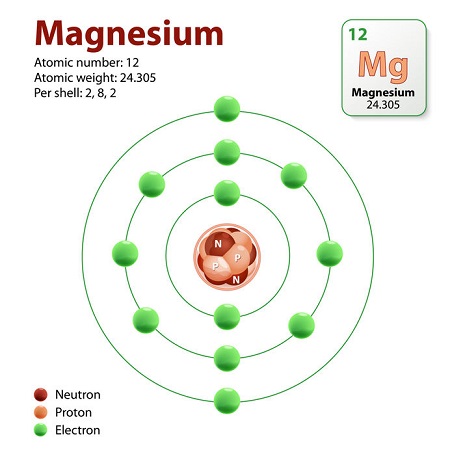
How Many Protons Does Magnesium Have?
Each atom of magnesium contains twelve protons. This information is readily available in any periodic table via the atomic number. The atomic number is also known as the proton number because it equals the number of protons in an element’s nucleus; magnesium’s atomic number is twelve.
Protons Remain Consistent
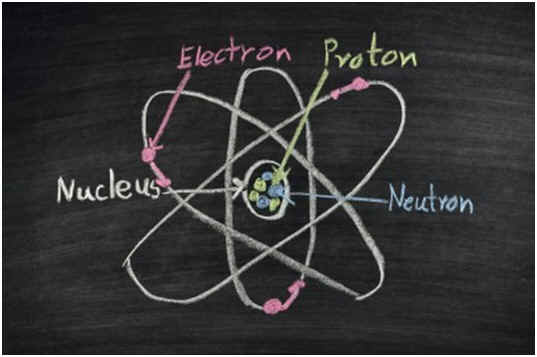
All elements always have the same number of protons, but confusion may arise from varying weights and charges on different compounds. For example, although magnesium has nine electrons when neutrally charged, in nature, it is often found with only ten. The number of neutrons in an element also may vary, leading to confusion about the number of protons. However, the number of protons in an atom of any element never changes because the number of protons per atom defines elements.
Magnesium Is a Reactive Metal

Magnesium is a highly reactive alkaline earth metal. The reactivity is due to its tendency to oxidize or give up electrons. This reactivity can be observed by igniting a magnesium rod and observing the extremely bright white flame emitted. In fact, this tendency to burn at extremely high temperatures (reaching over 5000 degrees Fahrenheit) led to magnesium’s use in the firebombing of Europe during World War II.
Today, magnesium is commonly used as an alloy in structures or a pure element in high-performance automobiles. The pure form of magnesium is similar in quality to aluminum. Due to its low weight and high strength, magnesium is increasingly being used in the production of modern aircraft.
Expert Opinion

Quote:
“The element magnesium is symbolized by Mg. The atom is presented as 24/12Mg and is called Magnesium-24. Because the atom has 12 protons, it must also have 12 electrons. The mass number gives the total number of protons and neutrons, which means that this atom has 12 neutrons (24-12=12).”
Source: Zumdahl, Steven S., and Donald J. DeCoste. Introductory Chemistry.
7th ed. Belmont, CA: Brooks/Cole, Cengage Learning, 2010. Print.
Resources
The University of Illinois Dept. of Physics – Q&A: Magnesium