Does Water Expand When It Freezes?
✅ Answer:
Yes, water expands when it freezes! Unlike most substances, water increases in volume as it turns from a liquid to a solid. This is because of the special way water molecules arrange themselves in ice.
🧭 Dive Deeper:
- What Happens When Water Freezes?
- Why Does Ice Take Up More Space?
- How Much Does Water Expand When Frozen?
- Real-Life Examples of Expanding Ice
- Why This Matters in Science and Nature
- 🎯 Final Thoughts
- 📚 References
What Happens When Water Freezes?
When water cools to 0°C (32°F), it begins to freeze—changing from a liquid to a solid. This process is called freezing or solidification.
In most substances, molecules move closer together when they freeze, making the solid smaller than the liquid. But water behaves in a special way.
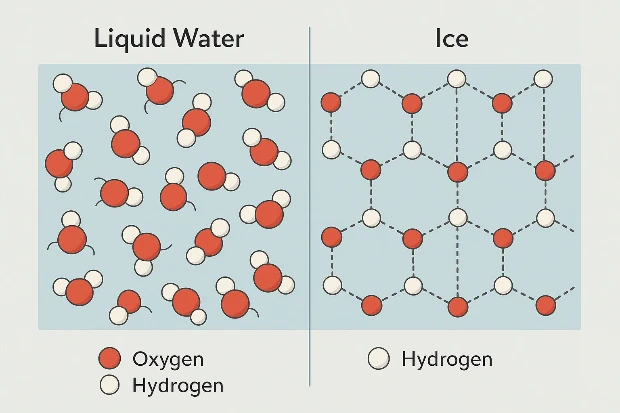
Why Does Ice Take Up More Space?
Water is made of two hydrogen atoms and one oxygen atom (H₂O). These molecules form hydrogen bonds—special connections that affect how water behaves when it cools down.
As water freezes:
- The molecules slow down and arrange themselves in a crystal-like pattern.
- This structure creates open spaces between the molecules.
- These spaces make ice less dense than liquid water.
That’s why ice floats and takes up more space than the water it came from.
🔬 Science Fact:
Water is one of the only substances that expands when it freezes. Most other liquids shrink into a denser solid form [1].
How Much Does Water Expand When Frozen?
Water expands about 9% in volume when it turns into ice.
| State | Volume Change |
|---|---|
| Liquid Water | Normal volume |
| Frozen Water | ~9% larger |
This may not sound like much, but it’s powerful enough to:
- Break glass containers
- Crack rocks
- Burst pipes in winter
📊 Interesting Stat:
Frozen water has a density of about 0.92 g/cm³, while liquid water is 1.00 g/cm³. That difference is what makes ice float [2].
Real-Life Examples of Expanding Ice
You might’ve seen water expanding when frozen in these ways:
- Ice cube trays: The ice rises above the original water line.
- Frozen soda cans: If left in the freezer, they explode due to expansion.
- Winter potholes: Water seeps into road cracks, freezes, and widens the gap.
- Burst pipes: In freezing weather, the water inside metal pipes expands and breaks them.
🧊 Fun Fact:
This expansion is also what helps create soil from rocks! Over time, freezing and thawing cause rocks to break apart—a process called frost weathering [3].
Why This Matters in Science and Nature
The fact that ice expands has big impacts:
- On Earth’s lakes and oceans: Ice floats, forming a protective top layer that insulates water below—allowing fish to survive winter.
- In weathering: Water expands in rock cracks, helping shape valleys and mountains over time.
- In plumbing and buildings: Engineers must account for freezing water when designing structures in cold areas.
🌍 Environmental Note:
Without water’s expansion, life in cold climates would look very different. Lakes could freeze solid, making survival for aquatic life nearly impossible.
🎯 Final Thoughts
So, does water expand when it freezes? Yes—it does! Water is unusual because it takes up more space as a solid than as a liquid. This is caused by the unique shape and bonding of water molecules when they freeze.
From frozen pipes to floating icebergs, this strange property of water plays a huge role in our daily lives and the natural world around us.
📚 References
- U.S. Geological Survey (USGS). “Water Density.”
https://www.usgs.gov/special-topics/water-science-school/science/water-density - National Snow & Ice Data Center. “The Science of Sea Ice.”
https://nsidc.org/learn/parts-cryosphere/sea-ice/science-sea-ice - BBC Bitesize. “Physical Weathering and Freeze-Thaw.”
https://www.bbc.co.uk/bitesize/guides/zt6r82p/revision/2
📌Learn More About Compound Water (H2O)
- What Is the Boiling Point of Water?💧What Your Kettle Can Teach You About Physics
- Does Water Expand When It Freezes? 💧Ice and Volume Explained
- Why Does Ice Float on Water? 💧 The Surprising Science of Frozen H₂O
- Why Does Water Have Surface Tension? 💧 The Invisible Skin on Every Drop
- Why Is Water Called the Universal Solvent? 💧 The Science of What It Can Dissolve