What Makes an Element a Metal?
Metals are everywhere—your bike, your coins, your phone, and even in your blood! But what actually makes an element a metal? Is it the shine? The weight? Or how it conducts electricity? In this article, we’ll break down what metals really are, how scientists classify them, and why they’re so important in both science and everyday life.
🔍 Dive Deeper
- What Is a Metal?
- Where Are Metals on the Periodic Table?
- Key Properties of Metals
- Why Do Metals Conduct Electricity?
- Types of Metals
- 🎯 Final Thoughts
- 📚 References
🔩 What Is a Metal?
A metal is a type of element that usually has the following traits:
- Shiny appearance (called luster)
- Good conductor of heat and electricity
- Malleable (can be bent or shaped)
- Ductile (can be stretched into wires)
- Solid at room temperature (except mercury)
Metals lose electrons easily, which makes them reactive in certain conditions. This ability to give up electrons is what helps them conduct electricity and form strong bonds in compounds.
🔬 Statistic: About 75% of all elements on the periodic table are metals [1].
Where Are Metals on the Periodic Table?
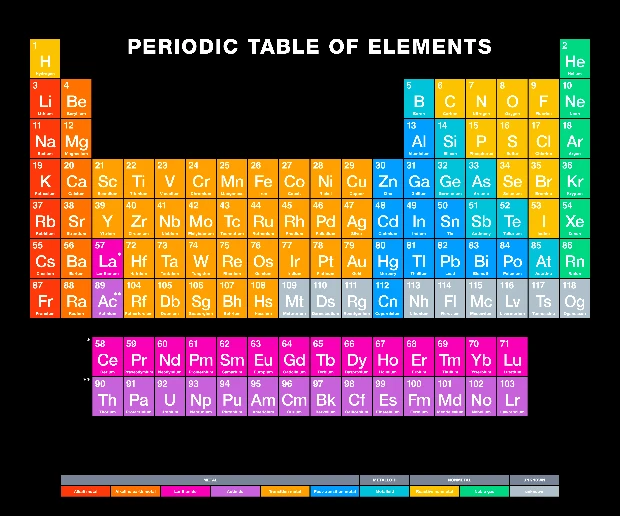
Metals are mostly found on the left and center of the Periodic Table. These include:
- Alkali metals (Group 1)
- Alkaline earth metals (Group 2)
- Transition metals (Groups 3–12)
- Post-transition metals (to the left of the staircase line)
The staircase line on the table helps divide metals from nonmetals. Elements touching that line are called metalloids, and they have mixed traits.
| Category | Location on Table | Examples |
|---|---|---|
| Alkali Metals | Group 1 | Sodium (Na), Potassium (K) |
| Transition Metals | Groups 3–12 | Iron (Fe), Copper (Cu) |
| Post-Transition | Near staircase | Lead (Pb), Tin (Sn) |
📊 Statistic: Transition metals make up 38 elements, including most of the metals used in industry [2].
🗝️Key Properties of Metals
Metals share a set of physical and chemical characteristics:
Physical Properties
- Shiny (luster): Reflect light well
- Ductile: Can be pulled into wires (like copper)
- Malleable: Can be hammered or shaped
- Conductive: Pass heat and electricity easily
- High melting points: Most metals melt at very high temperatures
Chemical Properties
- Reactive with acids
- Tend to form positive ions
- Corrode or rust over time (especially iron)
⚠️ Statistic: Iron rusts when exposed to oxygen and moisture, causing billions of dollars in damage each year worldwide [3].
⚡Why Do Metals Conduct Electricity?
Metals have “free electrons” that move easily from atom to atom. This is called the electron sea model. Because of these loose electrons:
- Heat moves quickly through metal
- Electricity flows easily
- Metals can bend without breaking
This is why copper is used in electrical wiring and aluminum is used in cooking pans.
⚡ Statistic: Silver is the best conductor of electricity, even better than copper—but it’s too expensive for most wiring uses [4].
💰Types of Metals
There are different categories of metals, each with unique uses:
| Type of Metal | Description | Common Uses |
|---|---|---|
| Alkali Metals | Very soft and reactive | Batteries, medicine |
| Transition Metals | Strong and less reactive | Tools, jewelry, construction |
| Noble Metals | Do not corrode easily | Gold, silver in electronics |
| Alloys (metal mixes) | Made by combining two or more metals | Steel (iron + carbon), bronze |
Some metals, like mercury, are liquid at room temperature, and others, like titanium, are extremely strong but lightweight.
🧠 Statistic: Titanium is as strong as steel but 45% lighter, making it perfect for aircraft and space shuttles [5].
🎯 Final Thoughts
What makes an element a metal? It’s not just about being shiny or hard—it’s about how the atoms behave. Metals are defined by their ability to lose electrons, conduct electricity, and form strong, useful materials. From coins to cars to cables, metals are key ingredients in our modern world. Knowing what makes an element a metal helps us understand chemistry, engineering, and even nature itself.
📚 References
- Royal Society of Chemistry. “What Is a Metal?”
https://edu.rsc.org - Los Alamos National Laboratory. “The Periodic Table of Elements.”
https://www.lanl.gov - NACE International. “The Global Cost of Corrosion.”
https://www.nace.org - Jefferson Lab. “Which Metals Conduct Electricity Best?”
https://education.jlab.org - Titanium Information Group. “Why Use Titanium?”
https://www.titaniuminfogroup.co.uk
📌 Learn More About Elements
- What Makes an Element Soluble?
- What Makes Up an Element?
- What Makes an Element Reactive?
- What Makes an Element a Metal?
- What Makes an Element Stable?
